The intriguing stability of the Carbon Monoxide molecule is intrinsically linked to the nature of the bond in carbon monoxide. Computational Chemistry methods provide a powerful means for analyzing the electronic structure and properties of this bond. Linus Pauling’s early contributions to understanding chemical bonding laid the groundwork for modern interpretations of the bond in carbon monoxide. Researchers at institutions like the National Institute of Standards and Technology (NIST) continue to explore the subtleties of this seemingly simple, yet complex, molecular system. Therefore, understanding the bond in carbon monoxide is significant.
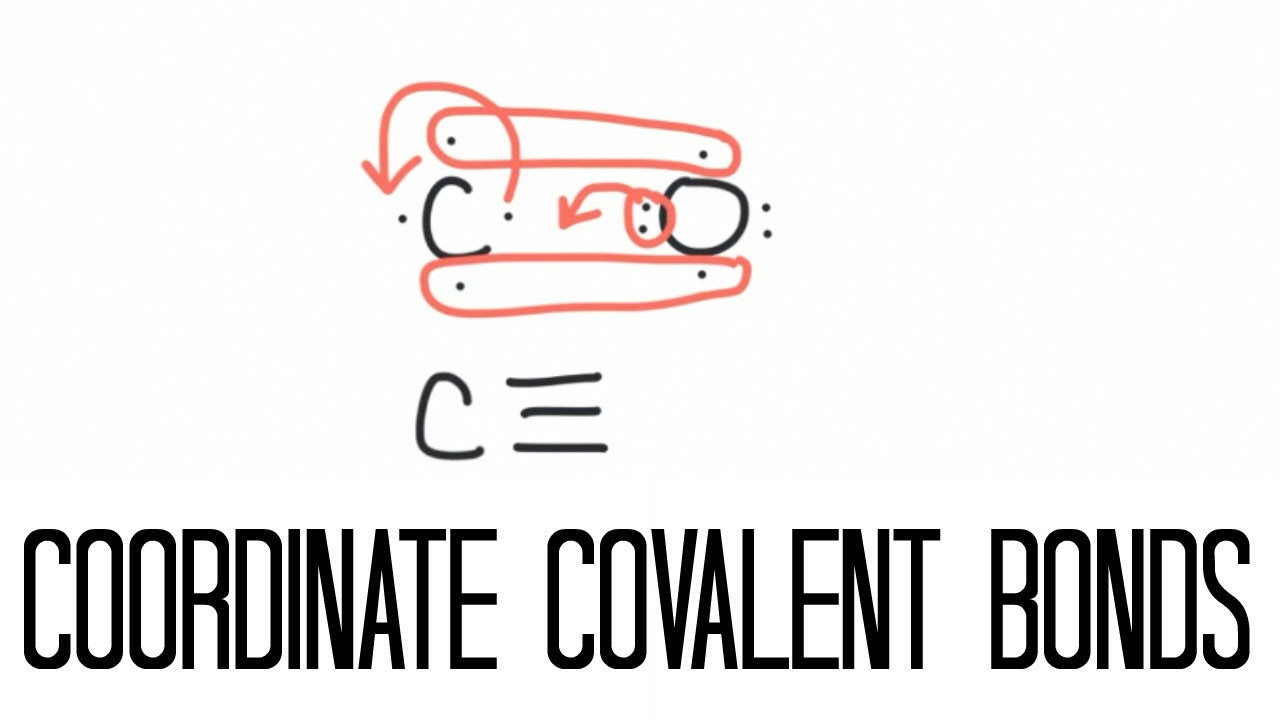
Image taken from the YouTube channel The Science Classroom , from the video titled Coordinate Covalent Bonding (Carbon Monoxide) .
Deconstructing the Bond in Carbon Monoxide: A Surprising Stability
The bond in carbon monoxide (CO) presents a unique case study in chemical bonding. Far from being a simple double bond as one might initially expect, the reality is a complex interplay of sigma and pi bonds, supplemented by a crucial dative interaction. Understanding this interplay explains the molecule’s exceptional stability and reactivity. The "surprising truth" lies in the fact that this seemingly simple diatomic molecule possesses a bond order approaching three and exhibits significant polarity despite both atoms having relatively high electronegativity.
The Initial Bonding Picture: Sigma and Pi Bonds
The Sigma Bond Framework
The initial foundation of the bond in carbon monoxide arises from the overlap of atomic orbitals to form sigma (σ) and pi (π) bonds. The carbon atom, with its electron configuration of 1s²2s²2p², contributes its 2s and 2p orbitals to bonding. Similarly, oxygen (1s²2s²2p⁴) contributes its 2s and 2p orbitals. The overlap of these atomic orbitals leads to the formation of one sigma bond. This sigma bond is formed primarily by the overlap of hybridized orbitals on carbon and oxygen.
The Pi Bond Contribution
Following the formation of the sigma bond, the remaining p orbitals on carbon and oxygen can overlap sideways to form pi (π) bonds. Two sets of p orbitals are oriented perpendicularly to the sigma bond axis, resulting in the formation of two pi bonds. This suggests a triple bond consisting of one sigma and two pi bonds – a common initial assumption.
The Crucial Role of Dative Bonding
What is Dative Bonding?
The key element that distinguishes the bond in carbon monoxide from a straightforward triple bond is the presence of a dative, or coordinate, covalent bond. In a dative bond, one atom donates both electrons to form the shared pair.
Carbon’s Lone Pair Donation
In carbon monoxide, carbon possesses a lone pair of electrons that reside in a non-bonding molecular orbital. Oxygen, being more electronegative, can accept these electrons into a vacant antibonding molecular orbital. This donation creates a dative bond from carbon to oxygen, increasing the overall bond order and further stabilizing the molecule. This donation also contributes significantly to the observed dipole moment of carbon monoxide.
Consequences of Dative Bonding
The dative bond has several critical consequences:
- Increased Bond Order: The dative bond effectively adds to the total bond order, making it closer to three than just two from the sigma and pi bonds alone. This explains the exceptionally strong bond in carbon monoxide.
- Polarity Modification: While oxygen is more electronegative, the dative bond partially compensates for this difference by transferring electron density from carbon to oxygen. This leads to a smaller than expected dipole moment, but a measurable one nonetheless with a negative charge on the carbon end.
- Enhanced Stability: The dative bond contributes significantly to the overall stability of the molecule. The resonance stabilization afforded by the combination of covalent and dative bonding makes carbon monoxide remarkably resistant to dissociation.
Molecular Orbital (MO) Theory Perspective
MO Diagram Overview
A more complete understanding comes from considering the molecular orbital (MO) diagram of carbon monoxide. This diagram illustrates how atomic orbitals combine to form bonding and antibonding molecular orbitals. The filling of these MOs dictates the overall bond order and stability.
Key MOs and Their Contributions
The key molecular orbitals contributing to the bond in carbon monoxide are:
- *σ2s and σ2s:* Formed from the 2s atomic orbitals. The bonding σ2s orbital is filled, while the antibonding σ2s orbital is also filled. The net effect is little contribution to the overall bond order.
- σ2p: A bonding sigma orbital formed from the 2p atomic orbitals. This contributes strongly to the overall sigma bonding framework.
- π2p: Two degenerate bonding pi orbitals formed from the 2p atomic orbitals. These contribute significantly to the pi bonding character.
- σ2p and π2p: Antibonding sigma and pi orbitals, respectively. These remain largely unoccupied in the ground state of carbon monoxide, indicating strong bonding character.
Bond Order Calculation and its Implications
Based on the MO diagram, the bond order can be calculated as:
(Number of electrons in bonding orbitals – Number of electrons in antibonding orbitals) / 2.
In carbon monoxide, this calculation yields a bond order close to three, reflecting the strong triple bond character, including the impact of the dative bond. The MO theory confirms the surprising stability and strong bond energy of carbon monoxide, highlighting the delocalization of electrons and the complex interplay of atomic orbitals in forming the molecular orbitals.
Table: Comparison of Bonding Contributions
| Bond Type | Orbital Overlap | Electron Contribution | Impact on Bond Order | Polarity Influence |
|---|---|---|---|---|
| Sigma (σ) | Head-on | 2 | +1 | Establishes framework, weak impact on polarity |
| Pi (π) | Sideways | 4 | +2 | Contributes to bond strength, reinforces framework. |
| Dative (C → O) | Donation | 2 | + ~0.5-1 | Shifts electron density towards oxygen |
Carbon Monoxide Bond: FAQs
Hopefully, the article has helped clarify the surprising nature of the carbon monoxide bond. Here are some frequently asked questions for further clarity:
Why is the carbon monoxide bond so strong despite carbon and oxygen having different electronegativity?
The strong bond in carbon monoxide arises from a combination of sigma and pi bonding, including a coordinate covalent bond from carbon to oxygen. This unique arrangement results in a very high bond order and exceptional bond strength, exceeding expectations based on electronegativity differences alone.
Is the carbon monoxide bond a simple triple bond like in nitrogen gas?
While often represented as a triple bond, the bond in carbon monoxide is more complex. It involves a dative or coordinate covalent bond where carbon donates an electron pair to oxygen. This donation contributes significantly to the overall bond strength and polarity.
How does the structure of the carbon monoxide bond influence its toxicity?
The electronic structure of the carbon monoxide bond makes carbon monoxide a good ligand that readily binds to the iron in hemoglobin. This binding prevents oxygen transport, leading to carbon monoxide poisoning.
Does the bond in carbon monoxide have any industrial importance?
Yes. The strong and reactive carbon monoxide bond is vital in many industrial processes, including the Fischer-Tropsch process for producing synthetic fuels and in the production of various chemicals and materials. Its unique bonding characteristics make it a valuable building block.
So, there you have it – a little deeper dive into the surprising truth about the bond in carbon monoxide. Hopefully, you found that interesting! Keep digging, and who knows what else you might uncover?