The molecular orbital theory elucidates the bonding characteristics of carbon monoxide (CO). Carbon, a tetravalent element, forms a triple bond with oxygen in CO, exhibiting a unique structure. Spectroscopic analysis performed by the National Institute of Standards and Technology (NIST) provides precise data on the bond length and vibrational frequencies pertinent to the structure of carbon monoxide. Understanding this structure is crucial for various applications in catalysis, where CO serves as a ligand.
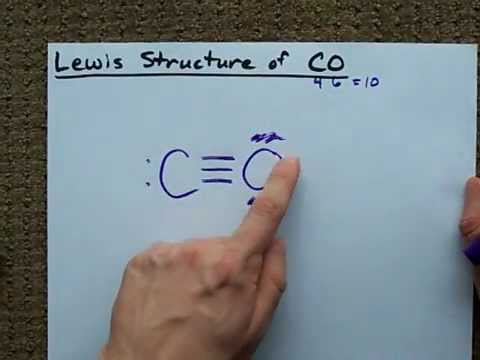
Image taken from the YouTube channel chemistNATE , from the video titled Lewis Structure of CO (Carbon Monoxide) .
Understanding the Structure of Carbon Monoxide
Carbon monoxide (CO) is a seemingly simple molecule with significant chemical properties and reactivity. Understanding its structure is crucial to understanding its behavior. This article will detail the structure of carbon monoxide, explaining its bonding characteristics, electronic configuration, and how these influence its properties.
Molecular Formula and Basic Composition
The molecular formula of carbon monoxide is CO, indicating that each molecule consists of one carbon atom and one oxygen atom. This simplicity belies a complex electronic structure and strong covalent bonding.
Lewis Structure and Bonding
Initial Representation
The initial Lewis structure of CO might appear straightforward, placing a single bond between carbon and oxygen and adding lone pairs to satisfy the octet rule. However, this structure does not accurately reflect the bonding situation.
Refined Lewis Structure and Formal Charges
- Carbon monoxide features a triple bond between the carbon and oxygen atoms. This means that three pairs of electrons are shared between the two atoms.
- One lone pair resides on the carbon atom, and another lone pair resides on the oxygen atom.
- This arrangement minimizes the formal charges on both atoms, leading to a more stable structure. The formal charge on carbon is -1 and the formal charge on oxygen is +1. Although nonzero, this representation better represents the bonding in CO than a structure with only single or double bonds.
Resonance Structures
While the above structure is the most accepted, CO can also be represented through resonance structures that emphasize the partial charges on each atom. These resonance structures illustrate the contribution of different bonding arrangements to the overall electronic distribution.
Molecular Orbital Theory and Electronic Configuration
Overview of Molecular Orbital Theory
Molecular orbital (MO) theory provides a more accurate description of the bonding in CO than simple Lewis structures. MO theory combines atomic orbitals to form molecular orbitals that extend over the entire molecule.
Formation of Sigma and Pi Molecular Orbitals
- Atomic orbitals of carbon and oxygen combine to form sigma (σ) and pi (π) molecular orbitals.
- σ orbitals are formed by the head-on overlap of atomic orbitals.
- π orbitals are formed by the sideways overlap of atomic orbitals.
Electronic Configuration Diagram
The electronic configuration of CO can be represented using a molecular orbital diagram. The diagram shows the relative energy levels of the molecular orbitals and the filling of these orbitals with electrons. A simplified representation of the key orbitals:
- σ2s: Bonding sigma orbital formed from the combination of 2s atomic orbitals.
- **σ2s***: Antibonding sigma orbital formed from the combination of 2s atomic orbitals.
- π2p: Bonding pi orbitals formed from the combination of 2p atomic orbitals. These are degenerate (have the same energy).
- σ2p: Bonding sigma orbital formed from the combination of 2p atomic orbitals.
- **π2p***: Antibonding pi orbitals formed from the combination of 2p atomic orbitals.
- **σ2p***: Antibonding sigma orbital formed from the combination of 2p atomic orbitals.
The filling order is generally, from lowest to highest energy: σ2s, σ2s, π2p, σ2p, π2p, σ2p*.
Bond Order Calculation
The bond order is calculated as: (Number of electrons in bonding orbitals – Number of electrons in antibonding orbitals) / 2. In CO, there are 10 electrons involved in bonding (2 in σ2s, 2 in σ2p, and 4 in π2p) and 2 in antibonding (σ2s*). Therefore, the bond order is (10-2)/2 = 4. This is slightly higher than a triple bond.
Polarity and Dipole Moment
Electronegativity Difference
Oxygen is more electronegative than carbon, meaning it has a greater tendency to attract electrons.
Direction of the Dipole Moment
Despite oxygen being more electronegative, the dipole moment of CO is actually very small and points towards the carbon atom. This counterintuitive observation arises from the complex interplay of the electronegativity difference and the electronic configuration of the molecule. The lone pair on carbon contributes significantly to this dipole moment.
Impact on Chemical Reactivity
The slight polarity influences the reactivity of CO. It can act as a ligand in coordination complexes, donating electrons to metal atoms.
FAQs: Understanding Carbon Monoxide’s Structure
Still curious about carbon monoxide (CO) and its unique structure? Here are some frequently asked questions to help clarify key concepts:
What makes the structure of carbon monoxide so unique?
The structure of carbon monoxide is unique because it features a triple bond between the carbon and oxygen atoms. This triple bond consists of one sigma bond and two pi bonds, making it exceptionally strong and stable. There is also a formal negative charge on the carbon and a formal positive charge on the oxygen.
Why is the carbon atom in CO negatively charged despite oxygen being more electronegative?
Although oxygen is more electronegative and usually attracts electrons, the formal charge calculation considers the number of valence electrons each atom "owns" in the molecule. In the structure of carbon monoxide, carbon formally "owns" more electrons than it should, leading to a negative formal charge, while oxygen has fewer than it should, leading to a positive formal charge.
Is carbon monoxide polar?
Yes, carbon monoxide is polar. Although the bond is mostly covalent, the unequal sharing of electrons due to the slight difference in electronegativity between carbon and oxygen creates a small dipole moment. This polarity contributes to the chemical properties of the structure of carbon monoxide.
How does the strong triple bond affect the reactivity of carbon monoxide?
The strong triple bond in the structure of carbon monoxide makes it relatively unreactive under normal conditions. However, it can participate in reactions with transition metals, where the metal can coordinate with the carbon atom and weaken the bond, facilitating further reactions, like those in catalytic converters.
So, there you have it – a peek into the fascinating world of carbon monoxide’s structure! Hopefully, you found this informative and maybe even a little mind-blowing. Keep exploring the wonders of chemistry; the more you learn about the structure of carbon monoxide and similar compounds, the more you’ll appreciate the intricate beauty of the molecular world.