Understanding the atom structure of copper is fundamental in materials science. Indeed, its unique arrangement dictates copper’s exceptional electrical conductivity, a property extensively utilized in electrical wiring. The Bohr model serves as a simplified yet crucial representation in visualizing the electron configuration, though more advanced computational methods like those employed at Lawrence Livermore National Laboratory provide a more precise depiction. Properties like copper’s ductility arise directly from these atomic interactions, making the subject area one of considerable interest for researchers, engineers and metallurgists alike.
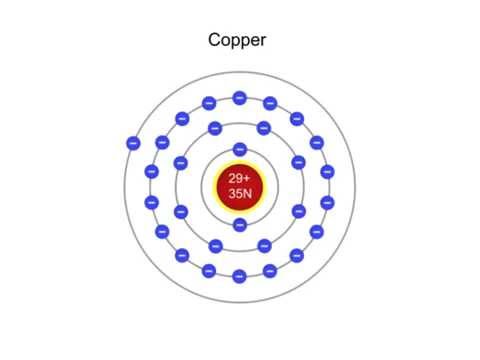
Image taken from the YouTube channel Engineering Technology Simulation Learning Videos , from the video titled What is a Copper Atom? .
Decoding Copper: A Deep Dive into its Atomic Structure
This article will explore the atom structure of copper, explaining its components and how they contribute to the metal’s unique properties. We will break down the fundamental particles within a copper atom and discuss their arrangement and behavior. The focus will remain on clarity and understanding, ensuring that readers gain a comprehensive grasp of copper’s atomic makeup.
Introduction to Copper and its Importance
Copper is a widely used metal, known for its excellent conductivity, malleability, and ductility. Understanding its atomic structure helps explain these valuable characteristics. We use copper in electrical wiring, plumbing, and various industrial applications.
- Key Uses: Electrical wiring, plumbing, coinage, industrial machinery.
- Important Properties: High electrical and thermal conductivity, malleability, ductility, corrosion resistance.
Understanding the atom structure of copper unlocks the understanding of why these properties exist.
The Basics of Atomic Structure
Defining the Atom
An atom is the basic building block of matter. It consists of a nucleus containing protons and neutrons, surrounded by electrons orbiting the nucleus.
Fundamental Particles: Protons, Neutrons, and Electrons
- Protons: Positively charged particles located in the nucleus. The number of protons defines the element.
- Neutrons: Neutral particles also located in the nucleus. They contribute to the atom’s mass and nuclear stability.
- Electrons: Negatively charged particles that orbit the nucleus in specific energy levels or shells.
The Atom Structure of Copper in Detail
Copper’s Atomic Number and Mass Number
Copper’s atomic number is 29. This means every copper atom has 29 protons in its nucleus. The most common isotope of copper has a mass number of 63.
- Atomic Number (Z): 29 (Number of protons)
- Mass Number (A): 63 (Total number of protons and neutrons)
- Number of Neutrons: 63 – 29 = 34 (Usually, but isotopes vary)
Electron Configuration of Copper
The arrangement of electrons around the nucleus is called the electron configuration. This configuration determines how copper interacts with other elements and its chemical properties. Copper is an interesting case due to its electron configuration:
- Expected Configuration: [Ar] 3d9 4s2
- Actual Configuration: [Ar] 3d10 4s1
This unusual configuration is because a full d-orbital is more stable than a partially filled one. An electron from the 4s orbital shifts to the 3d orbital, resulting in a more stable configuration.
Electron Shells and Orbitals
Electrons are arranged in shells around the nucleus. Each shell can hold a specific number of electrons. Within each shell, electrons occupy different orbitals.
- Shells: K, L, M, N (corresponding to principal quantum numbers n=1, 2, 3, 4, respectively)
- Orbitals: s, p, d, f (describing the shape of the electron cloud)
For copper:
| Shell | Orbitals | Number of Electrons |
|---|---|---|
| K (n=1) | 1s | 2 |
| L (n=2) | 2s, 2p | 8 |
| M (n=3) | 3s, 3p, 3d | 18 |
| N (n=4) | 4s | 1 |
Valence Electrons and Bonding
Copper has one valence electron in its outermost (4s) shell. This valence electron is responsible for copper’s ability to form bonds with other atoms, especially in metallic bonding. Metallic bonding, the sharing of electrons in a "sea," contributes to its high electrical and thermal conductivity.
Isotopes of Copper
Isotopes are atoms of the same element (same number of protons) but with different numbers of neutrons. Copper has two stable isotopes:
- Copper-63 (63Cu): Contains 29 protons and 34 neutrons. Approximately 69% abundance.
- Copper-65 (65Cu): Contains 29 protons and 36 neutrons. Approximately 31% abundance.
Other isotopes exist but are unstable (radioactive). These are used in medical imaging and other scientific applications, but do not contribute to the properties of bulk copper.
How Atom Structure Relates to Copper’s Properties
The atom structure of copper directly explains its unique properties:
- High Conductivity: The single valence electron in the 4s orbital is easily mobile, allowing electrons to flow freely through the metal, enabling excellent electrical and thermal conductivity. The complete 3d shell also contributes to this property by providing a path for electron flow.
- Malleability and Ductility: The metallic bonds in copper are non-directional, which means the atoms can slide past each other without breaking the bonds, allowing copper to be easily shaped and drawn into wires.
- Corrosion Resistance: While copper can corrode, it forms a protective oxide layer (patina) on its surface, which prevents further corrosion. This is because the copper atoms form a stable compound with oxygen.
Frequently Asked Questions About Copper’s Atom Structure
Here are some common questions regarding the atom structure of copper and its properties.
What makes copper a good conductor of electricity?
Copper’s excellent conductivity stems from its atom structure of copper. Specifically, it has one loosely held electron in its outermost shell. This "free" electron moves easily when voltage is applied, enabling electrical current to flow.
How is the atom structure of copper organized?
The atom structure of copper consists of a nucleus containing protons and neutrons. Orbiting the nucleus are electrons arranged in specific energy levels or shells. Copper has 29 protons, and a typical isotope has 34 neutrons in its nucleus, with its electrons arranged in shells around the nucleus.
What determines copper’s atomic number?
The atomic number of an element, including copper, is determined by the number of protons in its nucleus. Since the atom structure of copper has 29 protons, its atomic number is 29. This number uniquely identifies it as copper.
How does the atom structure of copper influence its color?
The characteristic reddish-orange color of copper is related to its atom structure. When light interacts with copper, electrons in its structure absorb and re-emit light at specific wavelengths. These wavelengths correspond to the color we perceive as copper.
So, there you have it! Hopefully, this breakdown of the atom structure of copper helped clarify things. Now you know a little bit more about why copper’s so awesome. Go impress your friends!