The formation of ionic bonds, a fundamental concept in chemistry, hinges significantly on electronegativity difference for ionic bond. The Pauling scale, developed by Linus Pauling, offers a method for quantifying this crucial difference. Atoms with large electronegativity difference for ionic bond, like those found in sodium chloride (NaCl), readily transfer electrons, resulting in strong electrostatic attractions. This process, vital to understanding material properties, demonstrates how electronegativity difference for ionic bond dictates the characteristics of many chemical compounds.
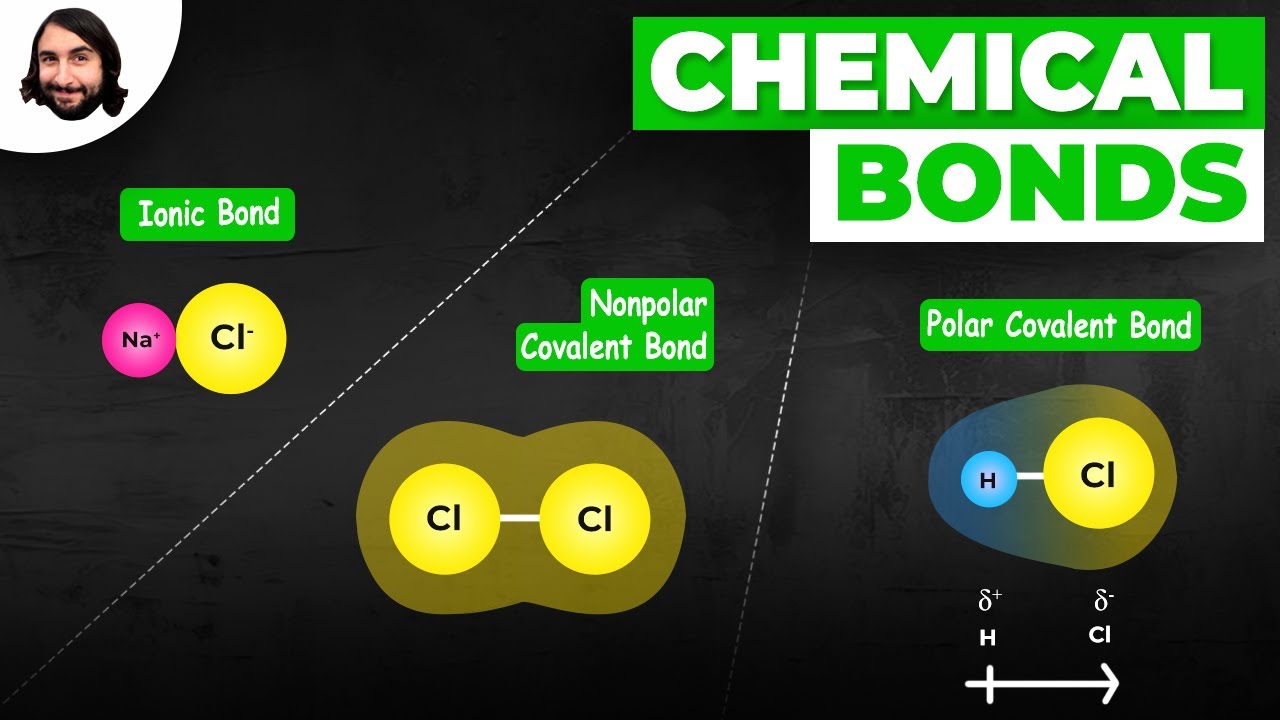
Image taken from the YouTube channel Professor Dave Explains , from the video titled The Chemical Bond: Covalent vs. Ionic and Polar vs. Nonpolar .
Ionic Bonds Decoded: Understanding the Role of Electronegativity Difference
The formation of ionic bonds is driven by a fundamental principle: the large electronegativity difference for ionic bond between two interacting atoms. This difference dictates the transfer of electrons, leading to charged ions and the strong electrostatic attraction that defines ionic compounds. This article explores this key concept and its implications.
What is Electronegativity?
Electronegativity is a measure of an atom’s ability to attract electrons to itself within a chemical bond. Linus Pauling developed a scale to quantify this property, with fluorine (F) being the most electronegative element (assigned a value of 4.0) and francium (Fr) the least electronegative.
- Electronegativity generally increases across a period (left to right) in the periodic table. This is because the nuclear charge increases, while the atomic radius decreases, leading to a stronger attraction for electrons.
- Electronegativity generally decreases down a group (top to bottom) in the periodic table. The increased number of electron shells leads to greater shielding of the nuclear charge, reducing the attraction for outer electrons.
Electronegativity Difference and Bond Type
The difference in electronegativity between two bonded atoms is a strong indicator of the type of chemical bond that will form. We can broadly categorize bond types based on this difference:
- Nonpolar Covalent Bonds: Small electronegativity difference (generally less than 0.4). Electrons are shared relatively equally between the atoms. Examples: H-H, C-H.
- Polar Covalent Bonds: Moderate electronegativity difference (generally between 0.4 and 1.7). Electrons are shared unequally, leading to partial charges (δ+ and δ-) on the atoms. Examples: H-Cl, O-H.
- Ionic Bonds: Large electronegativity difference (generally greater than 1.7). Electrons are effectively transferred from one atom to the other, forming ions with full charges (positive cation and negative anion). Examples: NaCl, MgO.
Numerical Ranges for Electronegativity Differences
While the ranges provided above are useful guidelines, it’s important to understand that they are not absolute rules. The "ionic character" of a bond increases as the electronegativity difference increases, and there is a gradual transition between polar covalent and ionic bonds.
| Bond Type | Electronegativity Difference (Approximate) |
|---|---|
| Nonpolar Covalent | 0 – 0.4 |
| Polar Covalent | 0.4 – 1.7 |
| Ionic | > 1.7 |
The Formation of Ionic Bonds: A Step-by-Step Process
The formation of an ionic bond involves the transfer of electrons from a less electronegative atom (typically a metal) to a more electronegative atom (typically a nonmetal).
- Electron Transfer: The atom with the lower electronegativity loses one or more electrons to the atom with the higher electronegativity. This results in the formation of a positively charged ion (cation) and a negatively charged ion (anion).
- Electrostatic Attraction: The oppositely charged ions are strongly attracted to each other due to electrostatic forces (Coulomb’s Law). This attraction is what holds the ions together in the ionic bond.
- Lattice Formation: In the solid state, ionic compounds form a crystal lattice structure, where each ion is surrounded by ions of the opposite charge. This arrangement maximizes the electrostatic attraction and minimizes the repulsion between ions of the same charge.
Examples Illustrating Electronegativity Difference for Ionic Bond
Let’s consider a few examples to illustrate how the electronegativity difference helps predict the formation of ionic bonds:
-
Sodium Chloride (NaCl): Sodium (Na) has an electronegativity of 0.93, and chlorine (Cl) has an electronegativity of 3.16. The electronegativity difference is 3.16 – 0.93 = 2.23. This large difference indicates that an ionic bond will form. Sodium loses an electron to become Na+, and chlorine gains an electron to become Cl-. These ions are then held together by electrostatic attraction.
-
Magnesium Oxide (MgO): Magnesium (Mg) has an electronegativity of 1.31, and oxygen (O) has an electronegativity of 3.44. The electronegativity difference is 3.44 – 1.31 = 2.13. This difference also indicates the formation of an ionic bond. Magnesium loses two electrons to become Mg2+, and oxygen gains two electrons to become O2-.
-
Water (H₂O): Hydrogen (H) has an electronegativity of 2.20, and oxygen (O) has an electronegativity of 3.44. The electronegativity difference is 3.44 – 2.20 = 1.24. This difference is within the range for polar covalent bonds. While the bond is polar, it is not considered ionic.
Factors Affecting Ionic Character
While electronegativity difference is a good predictor, other factors can influence the ionic character of a bond:
- Size of Ions: Larger ions tend to form more polarizable bonds, which may decrease the ionic character.
- Charge of Ions: Higher charges on ions lead to stronger electrostatic attraction and increase the ionic character.
FAQs: Ionic Bonds Decoded
This FAQ section addresses common questions about ionic bonds and the role of electronegativity difference in their formation. Hopefully, these answers will clarify any remaining confusion.
What exactly makes a bond "ionic"?
An ionic bond forms when electrons are transferred from one atom to another, creating oppositely charged ions that are strongly attracted to each other. The key factor determining whether a bond is ionic is the electronegativity difference between the atoms involved.
How much electronegativity difference is needed for an ionic bond?
Generally, an electronegativity difference of 1.7 or greater is considered indicative of an ionic bond. This substantial difference ensures a near-complete transfer of electrons. The larger the electronegativity difference, the more ionic character the bond possesses.
Can a bond be purely ionic?
In reality, no bond is perfectly ionic. Even in compounds with a large electronegativity difference, there is always some degree of covalent character. The concept of "ionic bond" is more of a classification on a spectrum. The high electronegativity difference for ionic bond shows a dominance on ionic behavior.
What happens if the electronegativity difference is less than 1.7?
If the electronegativity difference is less than 1.7, the bond is considered polar covalent or nonpolar covalent, depending on how close the electronegativity values are. In these cases, the electrons are shared, not transferred. A smaller electronegativity difference will be a smaller polarity bond.
Hopefully, you now have a much better understanding of electronegativity difference for ionic bond! Go forth and bond…ionically, that is!