The quantum mechanical model describes atoms using sophisticated mathematical equations, and understanding those solutions helps us grasp chemical behavior. Specifically, the complex shapes of orbitals in f subshell significantly impact how elements like Lanthanides and Actinides interact. Computational chemistry, a powerful tool, visualizes these orbitals, providing insights into the electron density distribution. This guide aims to demystify these shapes, providing a visual and conceptual understanding of how orbitals in f subshell contribute to chemical properties.
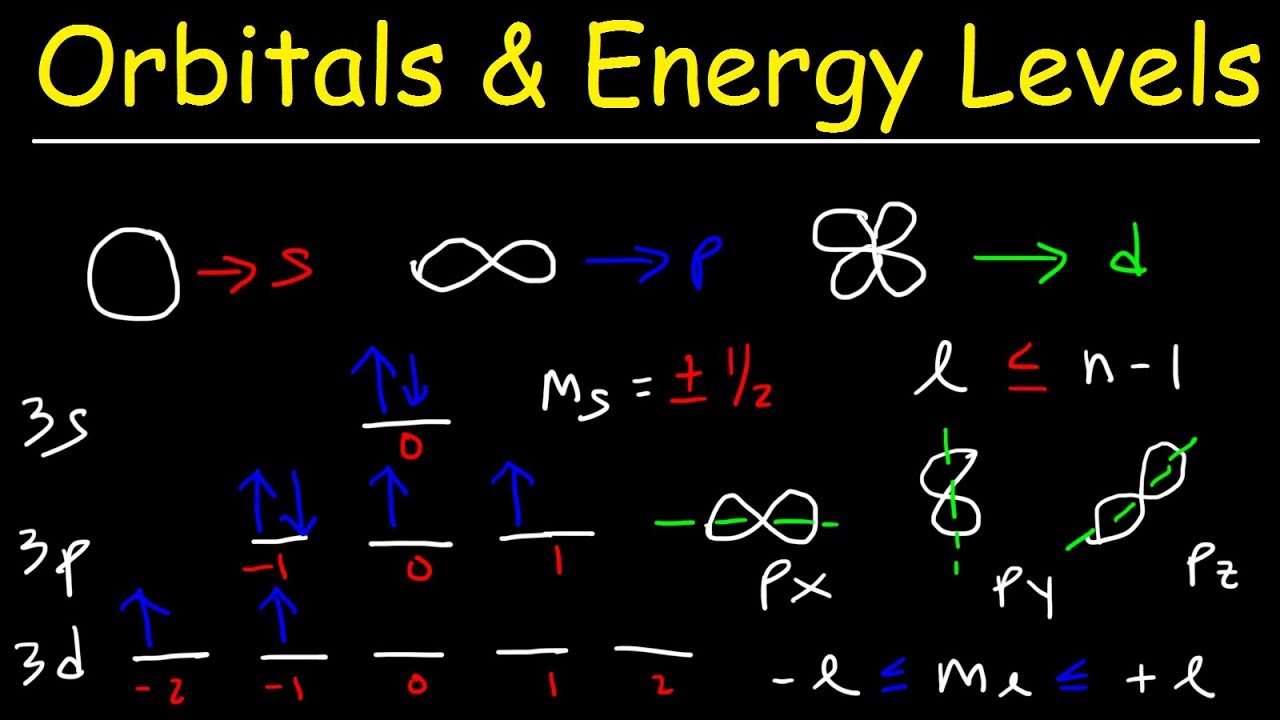
Image taken from the YouTube channel The Organic Chemistry Tutor , from the video titled Orbitals, Atomic Energy Levels, & Sublevels Explained – Basic Introduction to Quantum Numbers .
At the heart of every chemical reaction, every material property, and every biological process lies the intricate dance of electrons within atoms. These electrons don’t simply orbit the nucleus like planets around a sun. Instead, they reside in specific regions of space known as atomic orbitals, probabilistic zones dictated by the laws of quantum mechanics. Understanding these orbitals is paramount to deciphering the behavior of matter itself.
Atomic Orbitals: The Foundation of Chemical Behavior
Atomic orbitals are three-dimensional regions around an atom’s nucleus where there is a high probability of finding an electron.
Each orbital is defined by a unique set of quantum numbers, which dictate its energy level, shape, and spatial orientation. These orbitals are not physical boundaries but rather mathematical constructs derived from solutions to the Schrödinger equation.
They represent the probability distribution of an electron’s location. Without knowledge of these orbitals, modern chemistry would cease to exist.
The Enigmatic F Subshell: A Realm of Complexity
Among the various types of atomic orbitals – s, p, d, and f – the f subshell stands out for its complexity and unique influence on the elements that possess them. Unlike the simpler spherical s orbitals or the dumbbell-shaped p orbitals, f orbitals exhibit intricate, multi-lobed shapes.
These unusual shapes contribute significantly to the distinctive properties of the lanthanides and actinides, elements where the f orbitals are being filled. The f subshell, appearing from the fourth energy level (n=4) onwards, consists of seven distinct f orbitals, each capable of holding two electrons, for a total of 14 electrons.
The presence of electrons in these f orbitals profoundly affects the chemical bonding, magnetic behavior, and spectroscopic properties of these elements.
Decoding the F Orbitals: A Visual Journey
This article embarks on a journey to unravel the complexities of f subshell orbitals.
Our aim is to provide a clear and intuitive understanding of their shapes, spatial orientations, and their impact on the behavior of atoms. We will delve into the quantum mechanical principles that govern their existence.
Through detailed explanations and visual aids, we seek to demystify these often-overlooked orbitals.
By the end, you will gain a deeper appreciation for their crucial role in shaping the world around us.
Atomic Orbitals: A Concise Review of Fundamental Concepts
Having set the stage for exploring the enigmatic f subshell, it’s crucial to solidify our understanding of the fundamental principles governing atomic orbitals themselves. Consider this a rapid yet thorough refresher on the core concepts that underpin all electronic behavior within atoms. With this base in place, we’ll be well-equipped to tackle the intricacies of the f orbitals and their unique characteristics.
Defining Atomic Orbitals: Probability and Space
At the most basic level, an atomic orbital represents a region of space surrounding an atom’s nucleus where there is a high probability of finding an electron.
It is imperative to remember that an orbital is not a fixed path or trajectory, but rather a probability distribution. It tells us where an electron is likely to be at any given moment, not precisely where it is.
This probabilistic nature arises from the wave-particle duality of electrons, a cornerstone of quantum mechanics.
Think of it like a blurry photograph of a rapidly moving object. The blur represents the area where the object is most likely to have been during the exposure.
Quantum Numbers: The Address of an Electron
Each atomic orbital is uniquely defined by a set of four quantum numbers, each providing critical information about the electron’s state:
-
Principal Quantum Number (n): Determines the energy level of the electron and corresponds to the electron shell (n = 1, 2, 3, etc.). Higher ‘n’ values indicate higher energy levels and greater distance from the nucleus.
-
Azimuthal Quantum Number (l): Also known as the angular momentum quantum number, defines the shape of the orbital and the subshell to which it belongs (l = 0, 1, 2, …, n-1). l = 0 corresponds to an s orbital, l = 1 to a p orbital, l = 2 to a d orbital, and l = 3 to an f orbital.
-
Magnetic Quantum Number (ml): Specifies the spatial orientation of the orbital in three-dimensional space (ml = -l, -l+1, …, 0, …, l-1, l). For example, a p subshell (l=1) has three p orbitals (ml = -1, 0, +1), oriented along the x, y, and z axes.
-
Spin Quantum Number (ms): Describes the intrinsic angular momentum of the electron, which is quantized and referred to as spin. It can only have two values: +1/2 (spin up) or -1/2 (spin down).
These quantum numbers are not arbitrary labels; they are solutions to the Schrödinger equation, a fundamental equation in quantum mechanics that describes the behavior of electrons in atoms.
A Brief Overview of s, p, and d Orbitals
Before diving into the f orbitals, let’s briefly recap the shapes and characteristics of the simpler s, p, and d orbitals:
-
s Orbitals: Spherical in shape, with the highest probability density at the nucleus. There is one s orbital per energy level.
-
p Orbitals: Dumbbell-shaped, with two lobes separated by a node at the nucleus. There are three p orbitals per energy level (starting from n=2), oriented along the x, y, and z axes.
-
d Orbitals: More complex shapes, often described as having four lobes. There are five d orbitals per energy level (starting from n=3), with various spatial orientations.
Understanding the fundamental shapes and spatial arrangements of s, p, and d orbitals provides a crucial foundation for visualizing and comprehending the even more complex shapes and orientations of the f orbitals. This foundational knowledge is essential for appreciating the unique influence of f electrons on the properties of elements that possess them.
Having established a solid foundation in atomic orbital theory and quantum numbers, we can now turn our attention to the captivating realm of the f subshell. It’s here that the elegance and complexity of atomic structure truly begin to unfold. Understanding the characteristics and significance of this subshell is paramount to grasping the behavior of heavier elements and their unique chemical properties.
Introducing the F Subshell: Characteristics and Significance
The f subshell represents a significant step up in complexity compared to the s, p, and d subshells. It’s characterized by its higher energy level, more intricate spatial arrangements, and a larger capacity for holding electrons. These factors collectively contribute to the distinctive properties observed in elements where the f subshell plays a dominant role.
The Sevenfold Nature of F Orbitals
Unlike the s subshell, which contains a single orbital, or the p subshell with its three orbitals, the f subshell boasts a total of seven distinct f orbitals.
Each of these orbitals corresponds to a specific spatial orientation, dictated by the magnetic quantum number (ml). The allowed values for ml in the f subshell range from -3 to +3 (-3, -2, -1, 0, 1, 2, 3), giving rise to the seven individual f orbitals.
These seven orbitals are degenerate, meaning they possess the same energy level in the absence of external fields.
Energy Levels and the F Subshell
The f subshell makes its grand entrance starting at the fourth energy level (n=4). This means that elements in the fourth period and beyond can potentially accommodate electrons in their f orbitals. However, it’s important to note that the filling of the f subshell doesn’t strictly follow the order of increasing principal quantum number (n).
Due to interelectronic repulsions and relativistic effects, the energies of the orbitals can shift. As a result, the 4f orbitals are not filled until after the 5s and 5p orbitals and even the 6s orbitals have been occupied.
This seemingly counterintuitive filling order leads to the unique electronic configurations of the Lanthanides (Ce-Lu) and Actinides (Th-Lr).
Significance in the Periodic Table
The presence of the f subshell and its gradual filling across the Lanthanide and Actinide series profoundly influences the chemical behavior of these elements. The f electrons are not as effective at shielding the outer electrons from the nuclear charge.
This leads to a phenomenon known as the Lanthanide contraction, where the ionic radii of the Lanthanide ions decrease steadily across the series. This contraction affects the properties of subsequent elements in the periodic table as well.
Moreover, the f electrons contribute to the magnetic and spectroscopic properties of these elements, making them valuable in various technological applications, from magnets to luminescent materials.
Having established a solid foundation in atomic orbital theory and quantum numbers, we can now turn our attention to the captivating realm of the f subshell. It’s here that the elegance and complexity of atomic structure truly begin to unfold. Understanding the characteristics and significance of this subshell is paramount to grasping the behavior of heavier elements and their unique chemical properties.
Quantum Mechanical Description: Understanding F Orbitals Through Equations
To truly understand the nature of f orbitals, we must delve into the realm of quantum mechanics. This framework provides the fundamental equations and principles that govern the behavior of electrons within atoms, ultimately dictating the shapes, energies, and spatial orientations of atomic orbitals.
The Central Role of Quantum Mechanics
Quantum mechanics is not just a theoretical abstraction; it is the essential tool for describing and predicting the behavior of electrons in atoms and molecules. Classical mechanics, which accurately describes the motion of macroscopic objects, fails when applied to the subatomic world. Quantum mechanics provides a more accurate and complete picture.
Its principles explain why electrons exist in specific energy levels, why they occupy particular regions of space around the nucleus, and how they interact with each other and with external fields.
The Schrödinger Equation: A Cornerstone of Atomic Theory
At the heart of quantum mechanics lies the Schrödinger equation. This equation, formulated by Erwin Schrödinger, is a mathematical expression that describes the time evolution of a quantum mechanical system.
For the hydrogen atom, with its single proton and single electron, the Schrödinger equation can be solved exactly. The solutions to this equation are a set of mathematical functions called wave functions, each corresponding to a specific energy level and orbital shape.
For atoms with multiple electrons, the Schrödinger equation becomes incredibly complex and cannot be solved exactly. However, various approximation methods can be used to obtain highly accurate solutions, providing valuable insights into the electronic structure of multi-electron atoms.
Wave Functions: Mapping Electron Behavior
Wave functions, denoted by the Greek letter psi (ψ), are the mathematical descriptions of electrons within an atom. They contain all the information about the electron’s state, including its energy, momentum, and spatial distribution.
The square of the wave function (|ψ|^2) gives the probability density of finding an electron at a particular point in space. This probability density is what we visualize when we depict atomic orbitals as cloud-like shapes. Where the density is high, the probability of finding an electron is high.
The shape of an atomic orbital is therefore a representation of the region of space where an electron is most likely to be found, as dictated by the wave function.
Quantum Numbers: Defining Orbital Characteristics
The solutions to the Schrödinger equation are characterized by a set of quantum numbers. These numbers are not arbitrary; they arise naturally from the mathematical constraints imposed by the equation and provide a complete description of an electron’s state within an atom.
Of particular relevance to understanding the shapes and spatial orientations of f orbitals are the angular momentum quantum number (l) and the magnetic quantum number (ml).
The angular momentum quantum number (l) determines the shape of the orbital. For f orbitals, l = 3, which corresponds to their complex, multi-lobed shapes.
The magnetic quantum number (ml) determines the spatial orientation of the orbital in space. For f orbitals, ml can take on seven values: -3, -2, -1, 0, 1, 2, and 3. Each of these values corresponds to a distinct f orbital, oriented differently in three-dimensional space. This explains why there are seven f orbitals within the f subshell.
Having navigated the intricate mathematical landscape of quantum mechanics, we now face the challenge of translating abstract equations into tangible visualizations. This is where we attempt to visualize the unseen – the shapes and orientations of the f orbitals.
Visualizing the Unseen: Shapes and Orientations of F Orbitals
The seven f orbitals present a significant challenge to our visual intuition. Unlike the simpler s and p orbitals, f orbitals boast complex, multi-lobed structures that defy easy representation. We will explore their shapes, spatial orientations, and the influence of nodes on electron behavior.
The Intricacy of F Orbital Shapes
Describing the shapes of the seven f orbitals is no simple task. They are far from the neat spheres of s orbitals or the dumbbell shapes of p orbitals.
Instead, f orbitals exhibit intricate, multi-lobed geometries. These shapes are a direct consequence of the higher angular momentum associated with the f subshell (l=3).
It’s important to acknowledge that accurately depicting these orbitals is difficult. Diagrams and 3D visualizations offer approximations, but they can struggle to capture the full complexity of the electron density distribution.
Decoding the Spatial Orientations
Each of the seven f orbitals possesses a unique spatial orientation. These orientations are dictated by the magnetic quantum number (ml), which can take on values from -3 to +3, including 0.
These orientations are often designated using labels like fxyz, fz3, fx(x2-3y2), fy(3×2-y2), fxz2, fyz2, and f(x2-y2)z. These notations describe the angular dependence of the wave function and indicate how the orbital is oriented relative to the x, y, and z axes.
It’s crucial to understand that these labels are shorthand representations. They describe the mathematical form of the orbital, not necessarily its easily visualized shape.
Visualizing these orientations requires careful consideration of the coordinate system and the relationships between the lobes.
The Role of Diagrams and 3D Visualizations
Given the complex nature of f orbitals, diagrams and 3D visualizations are invaluable tools. These representations, while approximations, offer insights into the shape and spatial arrangement of the orbitals.
3D visualizations, in particular, can help to convey the three-dimensional nature of the electron density distribution. By rotating and manipulating these models, one can gain a better understanding of the orbital’s overall form.
However, it’s vital to remember that these are models. They represent the probability of finding an electron in a given region of space, not the electron’s exact location.
Nodes: Sculpting Electron Distribution
A crucial feature of f orbitals is the presence of nodes. Nodes are regions where the probability of finding an electron is zero. They can be either radial (spherical) or angular (planar or conical).
Radial nodes occur at specific distances from the nucleus, while angular nodes are defined by angles relative to the axes. The number and type of nodes are determined by the principal and angular momentum quantum numbers.
The presence of nodes has a significant impact on both the electron distribution and the energy of the orbital. They effectively divide the orbital into regions of positive and negative wave function amplitude, influencing the electron’s behavior.
The more nodes an orbital possesses, the higher its energy level. This is because the electron must have greater kinetic energy to "squeeze" into the regions between the nodes.
By understanding the shapes, orientations, and nodal properties of f orbitals, we gain a deeper appreciation for the complex and fascinating world of atomic structure.
Having navigated the intricate mathematical landscape of quantum mechanics, we now face the challenge of translating abstract equations into tangible visualizations. This is where we attempt to visualize the unseen – the shapes and orientations of the f orbitals.
Electron Configuration and F Orbitals: Filling the Subshell
The arrangement of electrons within an atom, known as its electron configuration, dictates its chemical behavior. Understanding how electrons populate atomic orbitals, especially the f orbitals, is crucial for predicting and explaining the properties of elements, particularly the lanthanides and actinides. The filling of these orbitals follows specific principles rooted in quantum mechanics.
Principles Governing Electron Configuration
The determination of an atom’s electron configuration relies on two fundamental principles: the Aufbau principle and Hund’s rule.
The Aufbau principle dictates that electrons first occupy the lowest energy orbitals available. This means starting with the 1s orbital and progressively filling higher energy levels.
However, the filling sequence isn’t always straightforward due to the overlap in energy levels between different subshells.
Hund’s rule provides further guidance, stating that within a given subshell (like the f subshell), electrons will individually occupy each orbital before doubling up in any one orbital.
This maximizes the total spin angular momentum and minimizes electron-electron repulsion, resulting in a more stable configuration.
Filling the F Subshell: A Maximum of Fourteen Electrons
The f subshell can accommodate a maximum of fourteen electrons, a consequence of its seven f orbitals, each capable of holding two electrons with opposite spins.
As we move across the periodic table, electrons progressively fill these f orbitals, leading to the unique electronic structures of the lanthanide and actinide series.
Lanthanides and Actinides: A Deep Dive into F Orbital Filling
The lanthanides (elements 57-71) and actinides (elements 89-103) are characterized by the filling of the 4f and 5f subshells, respectively. This process profoundly impacts their chemical properties.
Lanthanides: Gradual Filling of the 4f Orbitals
In the lanthanide series, the 4f orbitals are gradually filled across the period.
However, the energy levels of the 4f orbitals are very close to those of the 5d and 6s orbitals, leading to some exceptions in the filling order.
For example, Gadolinium (Gd) has a half-filled 4f subshell (4f7), which confers extra stability.
Actinides: Complexity and Radioactive Considerations
The actinides present a more complex picture. The filling of the 5f orbitals is less regular than that of the 4f orbitals in the lanthanides due to the even smaller energy differences between the 5f, 6d, and 7s orbitals.
Furthermore, many actinides are radioactive, making their study and characterization challenging. The interplay between relativistic effects and electron correlation also contributes to the complexity of their electronic structures.
Chemical Properties and F Orbital Configuration
The electron configuration of the f subshell directly influences the chemical properties of the lanthanides and actinides.
The lanthanides, with their similar outer electron configurations, tend to exhibit similar chemical behavior, primarily forming trivalent ions (3+).
The filling of the 4f orbitals, however, leads to subtle variations in ionic radii and magnetic properties across the series, which are exploited in various applications.
The actinides, with their more variable oxidation states and complex electronic structures, exhibit a wider range of chemical behaviors.
The involvement of the 5f orbitals in bonding can lead to the formation of unique complexes and compounds with interesting properties.
Having navigated the intricate mathematical landscape of quantum mechanics, we now face the challenge of translating abstract equations into tangible visualizations. This is where we attempt to visualize the unseen – the shapes and orientations of the f orbitals.
Probability Density: Mapping Electron Location in F Orbitals
While visualizing the shapes of f orbitals offers a valuable glimpse into their spatial characteristics, it’s crucial to understand that these representations are, in essence, depictions of probability density. Probability density provides a more nuanced understanding of where electrons are likely to be found around the nucleus. It is a critical concept for interpreting atomic behavior.
Understanding Probability Density
Probability density, denoted by Ψ², is the square of the wave function (Ψ).
It represents the probability of finding an electron within a given volume of space at a particular instant.
In simpler terms, it’s a map that indicates where the electron is most likely to be located at any given moment. A high probability density in a certain region means that the electron spends more time in that area.
It does not mean the electron is confined to that region; it simply implies a higher likelihood of its presence there.
Visualizing Electron Distribution: Probability Density Plots
Probability density plots offer a powerful way to visualize electron distribution within an atom.
These plots use varying shades or colors to represent different probability densities, effectively creating a three-dimensional map of electron location.
Regions with higher probability density appear more intensely colored, indicating a greater likelihood of finding the electron in that area.
By examining these plots, we can gain insights into the spatial distribution of electrons in f orbitals and better understand their behavior.
The Shape of F Orbitals: Reflecting Probability Density
The shapes we commonly associate with f orbitals are, in fact, a visual representation of the regions where the probability density is highest.
These shapes aren’t the actual paths of electrons, but rather the boundaries enclosing the volume where the electron is most likely to be found a significant percentage of the time (e.g., 90% or 95%).
The complex, multi-lobed shapes of f orbitals reflect the intricate probability density distribution dictated by their wave functions.
Each lobe corresponds to a region of high probability density, revealing the distinctive spatial characteristics of each f orbital and influencing the chemical behavior of elements containing them.
Having mapped the landscape of probability densities and visualized the potential whereabouts of electrons within f orbitals, we now turn our attention to the practical implications of these seemingly abstract concepts. The unique characteristics of f orbitals significantly influence the behavior of elements, especially those within the lanthanide and actinide series, and underpin a wide array of technological applications.
Significance and Applications: Where F Orbitals Matter
The influence of f orbitals extends beyond theoretical models, shaping the chemical properties of elements and driving innovations across diverse fields. Their impact is particularly pronounced in chemical bonding, especially within complexes formed by lanthanides and actinides, and in various technological applications leveraging their unique properties.
F Orbitals in Chemical Bonding: Lanthanide and Actinide Complexes
The partially filled f orbitals of lanthanides and actinides play a crucial role in their complex formation. Unlike transition metals, where d orbitals are directly involved in bonding, the f orbitals are more shielded from the surrounding ligands.
This shielding leads to several distinctive characteristics in their complexes:
-
High Coordination Numbers: Lanthanides and actinides can accommodate a large number of ligands around the central metal ion due to the less directional nature of f orbitals and reduced ligand-ligand repulsion.
-
Ionic Character: The bonding in these complexes tends to be more ionic than covalent, a consequence of the poor overlap between the shielded f orbitals and the ligand orbitals.
-
Weak Ligand Field Effects: The shielding also results in weak ligand field splitting of the f orbitals, leading to smaller energy differences between the f orbital energy levels. This affects their spectroscopic and magnetic properties.
The unique bonding characteristics imparted by f orbitals are crucial in applications such as nuclear fuel processing (actinides) and magnetic resonance imaging contrast agents (lanthanides).
Diverse Applications of F-Block Elements
The unique electronic configurations of lanthanides and actinides, stemming from their f orbitals, enable a plethora of applications across different fields.
Catalysis
Lanthanide compounds, particularly oxides, are used as catalysts in various chemical reactions, including:
-
Cracking of petroleum: Lanthanide-containing catalysts enhance the efficiency of breaking down large hydrocarbon molecules into smaller, more useful ones.
-
Three-way catalytic converters: Cerium oxide plays a critical role in reducing harmful emissions from vehicles by catalyzing the oxidation of carbon monoxide and hydrocarbons and the reduction of nitrogen oxides.
Magnetism
The unpaired electrons in the f orbitals of lanthanides give rise to strong magnetic moments. This property is exploited in:
-
Permanent magnets: Neodymium magnets, composed of neodymium, iron, and boron, are widely used in electric motors, hard disk drives, and wind turbines due to their high magnetic strength.
-
Magnetic resonance imaging (MRI): Gadolinium complexes are used as contrast agents to enhance the visibility of internal organs and tissues during MRI scans.
Luminescence
Certain lanthanide ions exhibit characteristic luminescence due to electronic transitions within their f orbitals. This is utilized in:
-
LED lighting: Europium and terbium compounds are used as phosphors in LED lights to produce red and green light, respectively.
-
Display technologies: Lanthanide phosphors are also employed in television screens and other display devices.
-
Security Inks: The sharp emission lines from lanthanide complexes can be used to create covert security markings in banknotes and other valuable documents.
Nuclear Technology
Actinides, specifically uranium and plutonium, are fundamental to nuclear power generation and nuclear weapons due to their ability to undergo nuclear fission.
-
Nuclear fuel: Uranium-235 is used as fuel in nuclear reactors to generate electricity through controlled nuclear fission.
-
Nuclear weapons: Plutonium-239 is a key component in nuclear weapons.
The applications highlighted above represent just a fraction of the ways in which f orbitals and the elements that possess them are shaping our world. Continued research and development promise even more innovative uses of these fascinating elements in the future.
F Subshell Orbitals: Frequently Asked Questions
This FAQ section aims to clarify common questions about the shapes and properties of f subshell orbitals. Let’s dive in.
What makes f subshell orbitals different from p or d orbitals?
F subshell orbitals have a more complex spatial arrangement compared to p or d orbitals. This is due to their higher angular momentum quantum number (l=3), resulting in more nodes and intricate shapes for the orbitals in f subshell. They also start appearing at a higher energy level (n=4).
How many orbitals are there in an f subshell?
There are seven orbitals in an f subshell. Each orbital holds a maximum of two electrons, meaning the f subshell can accommodate a total of 14 electrons. These seven orbitals have distinct spatial orientations and are labeled with ml values ranging from -3 to +3.
Why are the shapes of f subshell orbitals often described as "complex"?
The "complex" description arises from the multiple lobes and nodal planes present in the f subshell orbitals’ three-dimensional shapes. Visualizing these orbitals requires considering their mathematical representation, which involves complex spherical harmonics. This gives rise to the intricate and often less intuitive forms of the orbitals in f subshell.
Where do f subshell orbitals exist in the periodic table?
F subshell orbitals begin to be filled in the lanthanide and actinide series of elements. These elements are typically located in the f-block of the periodic table, which is usually positioned separately below the main body of the table. The filling of these orbitals accounts for many of the unique chemical properties observed in these elements as electrons occupy the orbitals in f subshell.
So, now you’ve got a handle on those tricky orbitals in f subshell! Hopefully, this visual guide made things a little clearer. Go forth and conquer those chemistry challenges!