VSEPR theory, a concept developed to predict the shapes of molecules, provides a foundational understanding for molecular and electron geometry. Understanding the principles of hybridization, especially the work of Linus Pauling in this area, helps explain the diverse shapes that molecules adopt. Examining molecules like methane (CH4) through simulations offered by educational websites like ChemEd DL allows learners to visualize the differences between electron and molecular geometry. Real-world applications in fields from drug design to materials science rely heavily on precise knowledge of molecular and electron geometry, making this understanding crucially important.
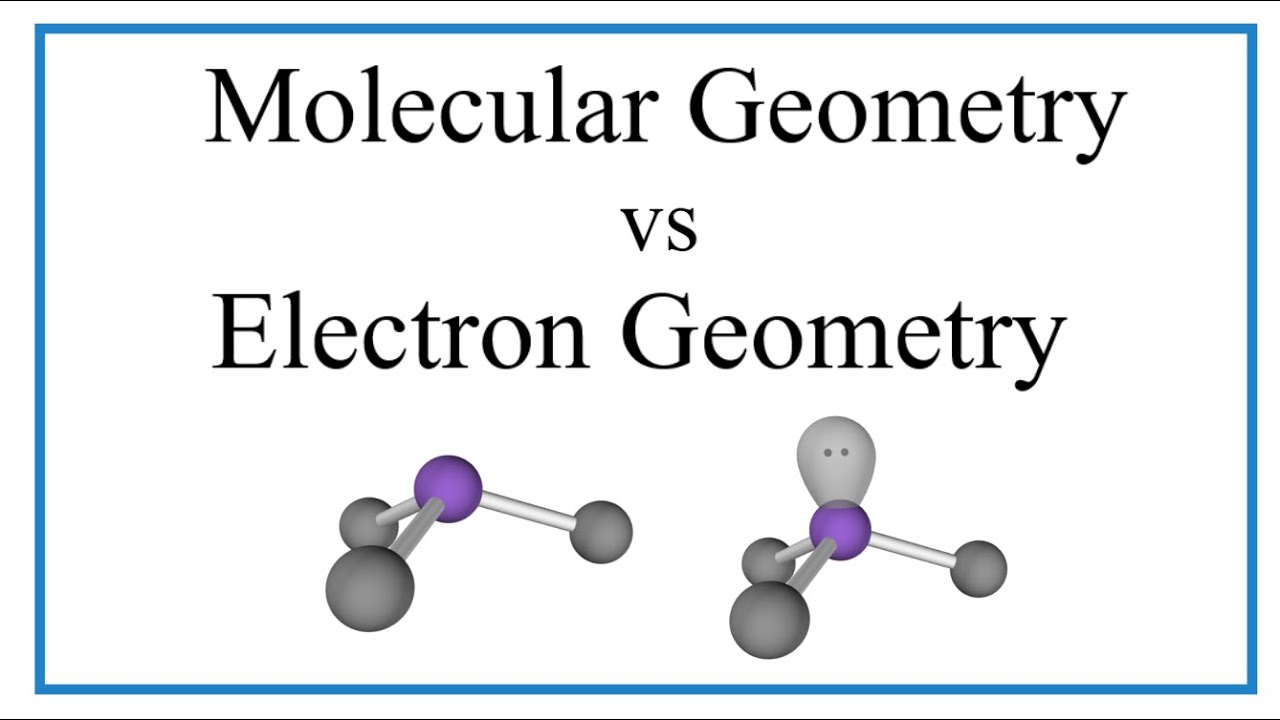
Image taken from the YouTube channel Wayne Breslyn (Dr. B.) , from the video titled Electron Geometry vs Molecular Geometry: Explanation & Examples .
Crafting the Ideal Layout for Your "Unlock Molecular Geometry" Article
To create a truly effective "Unlock Molecular Geometry: The Ultimate Visual Guide" article, specifically focusing on "molecular and electron geometry," a structured and visually appealing layout is crucial. The goal is to make a complex topic accessible and understandable to a broad audience. Here’s a breakdown of the best approach:
1. Introduction: Setting the Stage
Begin with a compelling introduction that immediately grabs the reader’s attention.
- Hook: Start with a real-world example or an intriguing question related to molecular shape and its impact (e.g., drug efficacy, material properties).
- Define Molecular and Electron Geometry: Clearly and concisely define both terms. Emphasize the key difference: electron geometry considers all electron pairs (bonding and lone pairs), while molecular geometry only considers the arrangement of atoms.
- State the Article’s Purpose: Explicitly state that the article will provide a visual and comprehensive guide to understanding and predicting molecular and electron geometries.
2. Foundational Concepts: Building a Solid Understanding
Before diving into specific geometries, establish the necessary foundation.
2.1. Valence Shell Electron Pair Repulsion (VSEPR) Theory
- Explain the Core Principle: Explain VSEPR theory in simple terms – electrons repel each other, and molecules arrange themselves to minimize this repulsion.
- Electron Domains: Define "electron domain" (a single bond, multiple bond, or lone pair all count as one electron domain).
- Visual Representation: Include a simple diagram illustrating electron domains around a central atom.
2.2. Representing Molecules: Lewis Structures
- Importance of Lewis Structures: Emphasize that drawing accurate Lewis structures is essential for predicting geometry.
- Brief Lewis Structure Guide: Provide a concise, step-by-step guide to drawing Lewis structures, focusing on common mistakes to avoid.
- Example Lewis Structure: Include a well-labeled example of a Lewis structure for a simple molecule, like water (H2O) or carbon dioxide (CO2).
3. Exploring Molecular and Electron Geometries: The Visual Guide
This is the heart of the article. Organize geometries systematically, using a tabular format for clarity and visual appeal.
3.1. Table Structure
Present the information in a table with the following columns:
| Electron Domains | Electron Geometry | Molecular Geometry | Bond Angle(s) | Example Molecule(s) | Visual Representation (Image/Diagram) | Notes/Special Cases |
|---|
3.2. Organizing the Table
- Start Simple: Begin with two electron domains and progressively increase the number of domains.
- Logical Progression: Within each "Electron Domains" category, start with cases where all domains are bonding pairs, then systematically introduce lone pairs.
- Include Visuals: This is critical. High-quality images or diagrams (preferably 3D renderings) showing both electron and molecular geometry for each example are essential. Use consistent coloring to represent atoms and lone pairs.
- Detailed Examples: The table should include the following (not exhaustive):
- 2 Electron Domains: Linear (CO2, BeCl2)
- 3 Electron Domains: Trigonal Planar (BF3), Bent (SO2)
- 4 Electron Domains: Tetrahedral (CH4), Trigonal Pyramidal (NH3), Bent (H2O)
- 5 Electron Domains: Trigonal Bipyramidal (PCl5), See-Saw (SF4), T-Shaped (ClF3), Linear (XeF2)
- 6 Electron Domains: Octahedral (SF6), Square Pyramidal (BrF5), Square Planar (XeF4)
- Bond Angles: Specify ideal bond angles and note deviations due to lone pair repulsion.
- Notes/Special Cases: Use this column to highlight exceptions, explain the impact of lone pairs on bond angles, or mention any unique characteristics of a particular geometry.
3.3. Emphasizing the Distinction
- Highlight the Difference: In the table and accompanying text, consistently reinforce the difference between electron and molecular geometry.
- Visual Cues: Use visual cues (e.g., shading, dotted lines) in the diagrams to distinguish between electron domains occupied by lone pairs and bonding pairs.
4. Advanced Topics (Optional, depending on target audience)
This section could be included if the goal is a more advanced guide.
4.1. Deviations from Ideal Bond Angles
- Lone Pair Repulsion: Explain how lone pairs exert a greater repulsive force than bonding pairs, leading to deviations from ideal bond angles.
- Multiple Bonds: Briefly discuss how multiple bonds can also influence bond angles, though generally to a lesser extent than lone pairs.
4.2. Predicting Geometry of Larger Molecules
- Focus on Central Atoms: Explain how to determine the geometry around each central atom in a larger molecule.
- Example: Include a more complex molecule and step through the process of determining the geometry around each key atom.
4.3. The Impact of Molecular Geometry
- Physical Properties: Connect molecular geometry to physical properties like polarity, boiling point, and solubility.
- Biological Activity: Briefly discuss how molecular shape influences biological activity, such as enzyme-substrate interactions.
FAQs: Understanding Molecular Geometry
This FAQ section clarifies key concepts from our "Unlock Molecular Geometry: The Ultimate Visual Guide," helping you grasp molecular and electron geometry principles more effectively.
What’s the difference between molecular and electron geometry?
Electron geometry considers all electron pairs (bonding and lone pairs) around the central atom. Molecular geometry only considers the arrangement of the atoms, ignoring lone pairs. This difference is crucial for predicting a molecule’s overall shape.
How do lone pairs affect molecular geometry?
Lone pairs repel bonding pairs more strongly than bonding pairs repel each other. This repulsion distorts the ideal bond angles predicted by electron geometry, leading to different and often smaller bond angles in the resulting molecular geometry.
Why is knowing molecular geometry important?
A molecule’s shape significantly influences its physical and chemical properties, including its polarity, reactivity, and interactions with other molecules. Understanding molecular geometry is key to understanding chemical behavior.
What is VSEPR theory and how does it help?
Valence Shell Electron Pair Repulsion (VSEPR) theory states that electron pairs around a central atom will arrange themselves to minimize repulsion. This theory is the foundation for predicting both electron and molecular geometry, providing a simple way to visualize and understand molecular shapes.
So, there you have it! Hopefully, this guide has made tackling molecular and electron geometry a little less daunting. Go forth and conquer those molecular structures!