Understanding the intricacies of potassium element protons neutrons electrons requires exploring several interconnected concepts. The periodic table, as organized by Dmitri Mendeleev, positions potassium prominently within the alkali metals, a group characterized by its high reactivity. This reactivity stems directly from the atom’s electronic configuration, a key focus in quantum mechanics, which dictates how potassium element protons neutrons electrons arrange themselves around the nucleus. Furthermore, the stable isotope of potassium, often analyzed using techniques developed by organizations like the International Atomic Energy Agency (IAEA), is defined by the specific number of protons and neutrons within its nucleus. Thus, a deep dive into potassium element protons neutrons electrons necessitates considering its placement on the periodic table, understanding fundamental quantum mechanical principles, and appreciating the role of isotopes and analytical techniques like those supported by the IAEA.
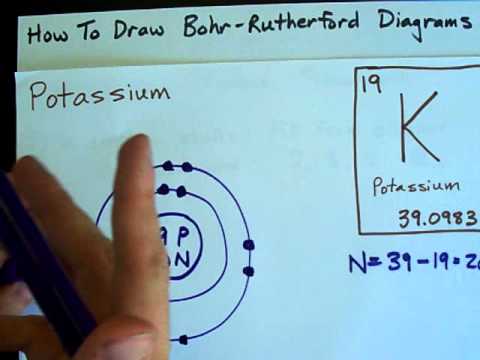
Image taken from the YouTube channel chemistNATE , from the video titled How to Draw Bohr-Rutherford Diagrams – Potassium .
Potassium: Unlocking the Secrets of Element’s Core!
This article delves into the atomic structure of potassium, focusing on the fundamental particles that define its properties and behavior. We will explore the composition of the potassium atom, specifically examining its protons, neutrons, and electrons. Understanding these components is key to grasping the element’s role in various chemical and biological processes.
Decoding the Potassium Atom: A Journey into the Nucleus
At the heart of every potassium atom lies the nucleus, the control center of the element. This nucleus is not empty; it’s densely packed with two types of particles: protons and neutrons. The interaction and number of these particles give potassium its identity.
The Role of Protons: Defining Potassium
Protons are positively charged particles residing within the nucleus. The number of protons dictates which element an atom is.
- Atomic Number: Potassium boasts an atomic number of 19. This means every potassium atom contains exactly 19 protons. Changing the number of protons transforms the atom into a different element altogether.
- Element Identity: Imagine you have an atom with 18 protons. That would be argon, not potassium! The proton count is non-negotiable for element definition.
Neutrons: The Neutral Stabilizers
Neutrons, as their name suggests, carry no electrical charge; they are neutral. These particles also reside in the nucleus alongside protons, contributing to the atom’s overall mass and influencing its stability.
- Isotopes: The number of neutrons can vary within potassium atoms, leading to different isotopes. Isotopes of potassium have the same number of protons (19) but different numbers of neutrons.
- Common Isotopes: The most common isotopes of potassium include potassium-39 (19 protons and 20 neutrons), potassium-40 (19 protons and 21 neutrons), and potassium-41 (19 protons and 22 neutrons).
Calculating the Mass Number
The mass number of an atom is the total number of protons and neutrons in its nucleus. For example:
| Isotope | Number of Protons | Number of Neutrons | Mass Number |
|---|---|---|---|
| Potassium-39 | 19 | 20 | 39 |
| Potassium-40 | 19 | 21 | 40 |
| Potassium-41 | 19 | 22 | 41 |
Electrons: Orbiting the Nucleus in Shells
Outside the nucleus, negatively charged particles called electrons orbit. These electrons are arranged in specific energy levels or shells around the nucleus.
Electron Configuration: The Arrangement of Electrons
The arrangement of electrons within these shells determines potassium’s chemical properties.
- Electron Shells: Electrons fill the shells closest to the nucleus first.
- Potassium’s Configuration: Potassium has 19 electrons. Its electron configuration is 2, 8, 8, 1. This means:
- The innermost shell holds 2 electrons.
- The second shell holds 8 electrons.
- The third shell holds 8 electrons.
- The outermost shell holds only 1 electron.
- Valence Electron: This single electron in the outermost shell is called the valence electron and is crucial for potassium’s reactivity.
The Drive for Stability: Why Potassium Reacts
Atoms "want" to have a stable outermost electron shell, typically containing 8 electrons (octet rule). Potassium, with its single valence electron, readily loses this electron to achieve a stable configuration.
- Ion Formation: By losing one electron, potassium forms a positive ion (K+). This positive charge results from having one more proton than electrons.
- Chemical Reactions: This tendency to lose an electron makes potassium highly reactive, meaning it readily combines with other elements to form compounds.
Connecting Protons, Neutrons, and Electrons: Potassium’s Properties
The interplay of protons, neutrons, and electrons dictates potassium’s physical and chemical characteristics.
- Reactivity: The ease with which potassium loses its valence electron contributes to its high reactivity.
- Physical State: Under standard conditions, potassium is a soft, silvery-white alkali metal.
- Atomic Weight: The average atomic weight of potassium is determined by the weighted average of the masses of its naturally occurring isotopes.
FAQs: Understanding Potassium
Here are some frequently asked questions about potassium, its properties, and its role in our world.
What makes potassium an element with unique properties?
Potassium’s unique properties stem from its atomic structure. As a chemical element, potassium has 19 protons in its nucleus, defining it as potassium. The number of electrons also equals 19 in a neutral potassium atom. The number of neutrons can vary leading to different isotopes of potassium.
Where can potassium be commonly found?
Potassium is abundant in nature. It’s found in various minerals, rocks, and soils. It’s also a vital electrolyte present in many foods, playing a crucial role in human health. Remember that the element potassium reacts readily with water.
How is potassium important for human health?
Potassium is essential for maintaining fluid balance, nerve function, and muscle contractions. As an element, it supports healthy blood pressure and helps regulate heart rhythms. Imbalances in potassium levels can lead to serious health problems.
How do the numbers of protons, neutrons, and electrons define potassium?
The number of protons in an atom’s nucleus identifies it as a specific element. Potassium has 19 protons. The number of neutrons can vary, resulting in isotopes. In a neutral potassium element, the number of electrons orbiting the nucleus is equal to the number of protons.
So there you have it – a glimpse into the fascinating world of potassium element protons neutrons electrons! Hopefully, this gives you a solid foundation for further exploration. Happy learning!