The stoichiometry plays a vital role in understanding the combustion of ethane equation. Ideal Gas Law further clarifies how the volume of the reactants and products directly influences this chemical process. The National Institute of Standards and Technology (NIST) provides invaluable data essential for accurately calculating the enthalpy changes during combustion of ethane equation. Effective manipulation of the ChemSketch software facilitates understanding the molecular structures involved in combustion of ethane equation, which is essential to mastering its balanced chemical form.
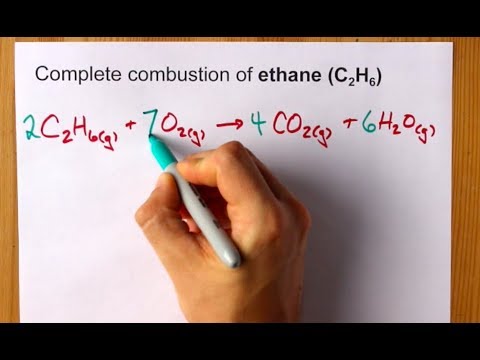
Image taken from the YouTube channel chemistNATE , from the video titled Complete Combustion of Ethane (C2H6) Balanced Equation .
Ethane, a simple hydrocarbon comprised of two carbon atoms and six hydrogen atoms (C2H6), stands as a significant player in the world’s energy landscape. Its utility spans across various applications, most notably as a versatile fuel source that powers industries and heats homes.
At the heart of ethane’s energy-releasing capabilities lies combustion, a fundamental chemical process that underpins much of modern civilization.
Combustion, in its essence, is a rapid chemical reaction between a substance with an oxidant, usually oxygen, to produce heat and light.
It’s the controlled burning of fuels like ethane that drives engines, generates electricity, and sustains countless industrial processes.
This guide embarks on a comprehensive exploration of the combustion of ethane equation, dissecting its components, balancing its elements, and revealing the underlying principles that govern this crucial reaction.
Ethane (C2H6): A Key Fuel Source
Ethane is a colorless, odorless gas that belongs to the alkane family of hydrocarbons.
It is primarily obtained as a byproduct of natural gas processing and petroleum refining.
Its abundance and relatively clean-burning properties make it a desirable fuel for various applications.
Beyond its use as a direct fuel, ethane serves as a vital feedstock in the petrochemical industry, where it is cracked into ethylene, a building block for plastics, resins, and other synthetic materials.
Combustion: The Essence of Energy Release
Combustion is far more than just burning; it’s a complex chemical transformation.
It involves the rapid oxidation of a fuel, releasing energy in the form of heat and light.
This energy release is what makes combustion such a valuable process.
The study of combustion encompasses a wide range of scientific disciplines, including chemistry, physics, and engineering.
Understanding the principles of combustion is crucial for designing efficient engines, minimizing pollution, and ensuring safe handling of flammable materials.
Deciphering the Combustion of Ethane Equation: A Guide’s Purpose
This guide serves as a comprehensive resource for understanding the combustion of ethane equation.
We will delve into the intricacies of this chemical reaction, breaking it down into manageable steps.
From identifying the reactants and products to balancing the equation and exploring the energy considerations, this guide will equip you with a solid foundation in the subject.
By the end of this exploration, you will not only be able to write and balance the combustion of ethane equation, but also appreciate the underlying scientific principles that govern this essential chemical process.
The Core Components: Reactants and Products
As we’ve established, the process of combustion is fundamental to understanding how ethane releases its stored energy. However, to truly grasp the combustion of ethane, we must first identify the key players in this chemical drama: the reactants and the products. These components dictate the reaction’s path and ultimately determine the energy released.
Identifying the Reactants: Ethane (C2H6) and Oxygen (O2)
Reactants are the substances that initiate and participate in a chemical reaction. In the case of ethane combustion, the primary reactants are, unsurprisingly, ethane itself (C2H6) and oxygen (O2).
Ethane: The Fuel Source
Ethane (C2H6), as we’ve already noted, is a hydrocarbon composed of two carbon atoms and six hydrogen atoms. It’s the fuel, the substance undergoing oxidation. Its chemical structure holds the potential energy that is unleashed during combustion. Ethane’s role is to provide the carbon and hydrogen atoms that will combine with oxygen to form new compounds.
Oxygen: The Oxidizer
Oxygen (O2) is the quintessential oxidizer, without which combustion cannot occur. It’s the driving force behind the reaction, readily accepting electrons from the ethane molecules. This electron transfer leads to the breaking of chemical bonds within ethane and the formation of new bonds with oxygen. The oxygen involved in ethane combustion is typically sourced from the air around us.
Unveiling the Products: Carbon Dioxide (CO2) and Water (H2O)
Products are the substances formed as a result of a chemical reaction. When ethane undergoes complete combustion, the primary products are carbon dioxide (CO2) and water (H2O). The formation of these specific products isn’t arbitrary; it’s dictated by the fundamental laws of chemistry.
Why Carbon Dioxide and Water?
During complete combustion, the carbon atoms in ethane combine with oxygen to form carbon dioxide (CO2), a greenhouse gas. Similarly, the hydrogen atoms in ethane react with oxygen to produce water (H2O), which is released as steam at the high temperatures of combustion.
The reason these specific products are formed lies in the drive for stability at the atomic level. Carbon and hydrogen atoms, when given the opportunity in an oxygen-rich environment, will naturally form these stable compounds, releasing energy in the process. If there is incomplete combustion, the carbon atoms can also form carbon monoxide (CO). This is caused by having too little oxygen in the reaction.
Unveiling the products and reactants involved in ethane combustion sets the stage for a more quantitative understanding of the process. The next crucial step involves translating this knowledge into a symbolic representation: the chemical equation. This equation serves as a concise summary of the chemical transformation that occurs during combustion.
Writing the Unbalanced Chemical Equation
The chemical equation is the language of chemistry. It concisely represents the transformation of reactants into products during a chemical reaction. Before we can delve into the intricacies of balancing the equation, we must first establish its initial, unbalanced form.
Presenting the Unbalanced Equation: A Foundation for Understanding
The unbalanced chemical equation for the combustion of ethane is:
C2H6 + O2 → CO2 + H2O
This equation tells us that ethane (C2H6) reacts with oxygen (O2) to produce carbon dioxide (CO2) and water (H2O).
However, it doesn’t tell us how much of each substance is involved. It simply identifies the participants in the reaction. This is why it’s considered unbalanced.
The Significance of Accurate Representation
The chemical equation, even in its unbalanced form, is not merely a symbolic representation. It is the foundation upon which all further calculations and analyses are built.
Accuracy in representing the reactants and products is paramount. An incorrect formula or a missing product can lead to flawed stoichiometric calculations and a misunderstanding of the entire combustion process.
Therefore, it is crucial to double-check and confirm the chemical formulas of all the reactants and products before proceeding.
The unbalanced equation serves as a preliminary roadmap.
It identifies the starting and ending points of the reaction.
It needs refinement through balancing to accurately reflect the quantitative relationships between the substances involved.
The unbalanced equation serves as a starting point, but it lacks the quantitative information necessary for accurate predictions about the reaction. To unlock the full potential of the chemical equation, we must balance it.
The Art of Balancing: Achieving Stoichiometric Precision
The cornerstone of chemical understanding lies in the balanced chemical equation. It’s more than just a symbolic representation; it’s a statement of quantitative relationships, underpinned by the fundamental principle of conservation of mass. Balancing ensures that we accurately reflect the stoichiometry of the reaction.
Understanding the Principle of Balancing Equations: Conservation of Mass
The act of balancing a chemical equation is rooted in the law of conservation of mass. This law dictates that matter cannot be created or destroyed in a chemical reaction. Therefore, the number of atoms of each element must be the same on both the reactant and product sides of the equation.
In essence, balancing is a process of adjusting coefficients—the numbers placed in front of each chemical formula—until the number of atoms for each element is equal on both sides. It is a process of iterative refinement to reflect accurate proportions.
Step-by-Step Guide to Balancing the Combustion of Ethane Equation
Balancing the combustion of ethane equation requires a systematic approach. Here’s a step-by-step guide:
-
Start with the most complex molecule: In this case, ethane (C2H6).
Begin by assuming one molecule of ethane. -
Balance carbon atoms: One molecule of C2H6 yields two carbon atoms. Thus, we need two molecules of CO2 on the product side:
C2H6 + O2 → 2 CO2 + H2O
-
Balance hydrogen atoms: One molecule of C2H6 contains six hydrogen atoms. To balance this, we need three molecules of H2O on the product side:
C2H6 + O2 → 2 CO2 + 3 H2O
-
Balance oxygen atoms: Now, count the oxygen atoms on the product side: (2 x 2) + (3 x 1) = 7 oxygen atoms.
To balance this on the reactant side, we need 3.5 molecules of O2:
C2H6 + 3.5 O2 → 2 CO2 + 3 H2O
-
Eliminate the fraction: To get rid of the fraction, multiply the entire equation by 2:
2 C2H6 + 7 O2 → 4 CO2 + 6 H2O
Detailed Instructions on Adjusting Coefficients
Adjusting coefficients is not a random process. It requires careful consideration of the atomic balance for each element. Here are some tips:
-
Treat polyatomic ions as single units if they appear unchanged on both sides of the equation.
-
Balance elements that appear in only one reactant and one product first.
-
Leave elements that appear in multiple compounds for last.
-
Always double-check your work to ensure all elements are balanced.
Demonstrate Balancing Equations of Ethane Combustion
Let’s walk through the balancing process again for clarity:
-
Unbalanced Equation: C2H6 + O2 → CO2 + H2O
-
Balancing Carbon: C2H6 + O2 → 2 CO2 + H2O
-
Balancing Hydrogen: C2H6 + O2 → 2 CO2 + 3 H2O
-
Balancing Oxygen: C2H6 + 3.5 O2 → 2 CO2 + 3 H2O
-
Eliminating Fraction: 2 C2H6 + 7 O2 → 4 CO2 + 6 H2O
The Balanced Chemical Equation: 2 C2H6 + 7 O2 → 4 CO2 + 6 H2O
The balanced chemical equation for the combustion of ethane is:
2 C2H6 + 7 O2 → 4 CO2 + 6 H2O
This equation tells us that two molecules of ethane react with seven molecules of oxygen to produce four molecules of carbon dioxide and six molecules of water.
This balanced equation is the foundation for all subsequent stoichiometric calculations.
Stoichiometry and its Relevance to Understanding the Equation
Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions. The balanced chemical equation provides the stoichiometric coefficients that allow us to predict the amount of reactants needed and products formed in a given reaction.
For example, the balanced equation tells us that for every 2 moles of ethane combusted, 4 moles of carbon dioxide are produced. This relationship is crucial for understanding the efficiency and impact of the combustion process.
The balanced equation serves as a starting point, but it lacks the quantitative information necessary for accurate predictions about the reaction. To unlock the full potential of the chemical equation, we must balance it.
Molar Matters: Quantitative Analysis
While balancing chemical equations gives us the ratios of reactants and products, it’s often necessary to understand how much of each substance is involved. This is where the concept of the mole comes into play, bridging the gap between the microscopic world of atoms and molecules and the macroscopic world of laboratory measurements. The mole allows us to perform quantitative analysis.
Understanding the Mole Concept
The mole is the SI unit for the amount of a substance. It’s defined as the amount of substance containing exactly 6.02214076 × 10²³ elementary entities (atoms, molecules, ions, etc.). This number is known as Avogadro’s number (Nᴀ).
Think of it like this: just as a "dozen" always means 12, a "mole" always means 6.022 x 10²³. It provides a convenient way to count enormous numbers of tiny particles.
The mole is crucial because it directly relates mass to the number of atoms or molecules. We use molar mass to determine the mass of one mole of a substance. Molar mass, expressed in grams per mole (g/mol), is numerically equivalent to the atomic or molecular weight of the substance.
Stoichiometry and Moles in Ethane Combustion
Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions.
Using the balanced chemical equation for ethane combustion (2 C2H6 + 7 O2 → 4 CO2 + 6 H2O), we can determine the molar ratios between all substances involved.
For example, the equation tells us that 2 moles of ethane react with 7 moles of oxygen to produce 4 moles of carbon dioxide and 6 moles of water. These ratios are essential for calculating the amount of product formed from a given amount of reactant or vice versa.
To calculate the amount of a substance required or produced in the ethane combustion reaction, you’ll need to perform the following steps:
- Convert the given mass of a substance to moles: Divide the mass of the substance (in grams) by its molar mass (in g/mol).
- Use the stoichiometric ratio from the balanced equation: Multiply the moles of the known substance by the appropriate mole ratio to find the moles of the desired substance.
- Convert the moles of the desired substance back to mass (if needed): Multiply the moles of the desired substance by its molar mass.
For example, if you want to determine the mass of carbon dioxide produced by the combustion of 10 grams of ethane, you would:
- Convert 10 grams of ethane to moles of ethane using its molar mass (30.07 g/mol).
- Use the stoichiometric ratio (4 moles CO2 / 2 moles C2H6) to find the moles of carbon dioxide produced.
- Convert the moles of carbon dioxide to grams of carbon dioxide using its molar mass (44.01 g/mol).
Avogadro’s Number: Connecting Moles to Molecules
Avogadro’s number (6.022 x 10²³) serves as the bridge between the macroscopic (moles) and the microscopic (individual molecules).
It tells us the exact number of molecules present in one mole of any substance. Therefore, if you know the number of moles of a substance, you can calculate the number of molecules present by multiplying the number of moles by Avogadro’s number.
Conversely, if you know the number of molecules, you can determine the number of moles by dividing by Avogadro’s number.
For example, if you calculated that the combustion of 10 grams of ethane produces 0.6 moles of carbon dioxide, you can determine the number of carbon dioxide molecules produced by multiplying 0.6 moles by Avogadro’s number, resulting in approximately 3.61 x 10²³ molecules of CO2.
This connection emphasizes the power of the mole concept, allowing us to translate between mass measurements and the actual number of molecules involved in the combustion process.
The balanced equation serves as a starting point, but it lacks the quantitative information necessary for accurate predictions about the reaction. To unlock the full potential of the chemical equation, we must balance it.
Molar Matters: Quantitative Analysis
While balancing chemical equations gives us the ratios of reactants and products, it’s often necessary to understand how much of each substance is involved. This is where the concept of the mole comes into play, bridging the gap between the microscopic world of atoms and molecules and the macroscopic world of laboratory measurements. The mole allows us to perform quantitative analysis.
Having established the quantitative relationships in ethane combustion, our focus now shifts to the energy dynamics of the reaction. Combustion, at its heart, is an energy-releasing process. Understanding the magnitude and nature of this energy release is crucial for both practical applications and theoretical insights.
Energy Considerations: Delving into Thermodynamics
Combustion reactions are not merely about transforming matter; they are fundamentally about energy transformation. Ethane combustion is a prime example of an exothermic reaction, where chemical energy is converted into thermal energy, manifested as heat and light.
Combustion as an Exothermic Reaction
An exothermic reaction is defined as a reaction that releases energy into its surroundings. This release of energy is due to the fact that the chemical bonds formed in the products (CO2 and H2O) are stronger and more stable than the bonds broken in the reactants (C2H6 and O2).
The excess energy, therefore, is liberated as heat. This release of energy is what makes ethane a valuable fuel source.
Enthalpy Change (ΔH) and Heat of Combustion
To quantify the energy released during ethane combustion, we use the concept of enthalpy change (ΔH). Enthalpy (H) is a thermodynamic property of a system, and the enthalpy change (ΔH) represents the difference in enthalpy between the products and reactants at constant pressure:
ΔH = H(products) – H(reactants)
For exothermic reactions, such as ethane combustion, ΔH is negative, indicating that the products have lower enthalpy (i.e., are more stable) than the reactants. This negative value signifies that energy is released to the surroundings.
The heat of combustion is specifically the amount of heat released when one mole of a substance undergoes complete combustion under standard conditions. It’s a crucial parameter in assessing the fuel efficiency and energy output of a combustion process.
Thermodynamics and Energy Transfer
Thermodynamics is the branch of physics that deals with energy and its transformations. The First Law of Thermodynamics, also known as the Law of Conservation of Energy, states that energy cannot be created or destroyed, only transformed from one form to another.
In ethane combustion, the chemical energy stored in the ethane and oxygen molecules is transformed into thermal energy (heat) and, to a lesser extent, light energy. The Second Law of Thermodynamics introduces the concept of entropy, which governs the direction of spontaneous processes.
Combustion reactions are irreversible processes that increase the entropy of the universe. This means that while energy is conserved, the quality of energy decreases as it is converted from a more ordered form (chemical energy) to a less ordered form (heat).
Understanding these fundamental thermodynamic principles is essential for optimizing combustion processes, minimizing energy waste, and developing more efficient energy technologies.
Combustion reactions are not merely about transforming matter; they are fundamentally about energy transformation. Ethane combustion is a prime example of an exothermic reaction, where chemical energy is converted into thermal energy, manifested as heat and light.
But what happens when the supply of oxygen is limited? Does the reaction proceed in the same manner? The answer lies in understanding the critical distinction between complete and incomplete combustion—a difference that dramatically alters the products formed and the energy released.
Complete vs. Incomplete Combustion: A Crucial Distinction
The combustion of ethane, like any hydrocarbon fuel, can proceed along two distinct pathways: complete combustion and incomplete combustion. The determining factor lies in the availability of oxygen during the reaction. Understanding these pathways is vital for optimizing energy production and minimizing harmful emissions.
Defining Complete Combustion
Complete combustion occurs when there is a sufficient supply of oxygen to fully oxidize the fuel. In the case of ethane (C2H6), complete combustion results in the formation of carbon dioxide (CO2) and water (H2O) as the sole products.
The balanced chemical equation for complete ethane combustion, as previously established, is:
2 C2H6 + 7 O2 → 4 CO2 + 6 H2O
This reaction releases the maximum possible amount of energy from the fuel, making it the desired outcome in most combustion applications.
The Perils of Incomplete Combustion
When the oxygen supply is limited, incomplete combustion takes place. Under these conditions, the fuel does not fully oxidize, leading to the formation of additional products besides carbon dioxide and water. These byproducts include carbon monoxide (CO), soot (elemental carbon, C), and unburned hydrocarbons.
The specific products formed and their relative amounts depend on the degree of oxygen deficiency.
Unlike the singular balanced equation for complete combustion, incomplete combustion can be represented by a range of equations, each reflecting a different degree of oxygen limitation and thus a different product distribution.
Comparing Products and Energy Yield
The differences between complete and incomplete combustion extend beyond just the products formed. They also have significant implications for energy yield and environmental impact.
Products:
- Complete Combustion: Primarily carbon dioxide (CO2) and water (H2O).
- Incomplete Combustion: Carbon monoxide (CO), soot (C), unburned hydrocarbons, in addition to CO2 and H2O.
Energy Yield:
- Complete Combustion: Releases the maximum possible energy stored in the fuel.
- Incomplete Combustion: Releases less energy compared to complete combustion, as some of the fuel’s chemical energy remains unreleased in the form of CO, soot, and unburned hydrocarbons.
Environmental and Health Concerns
Incomplete combustion poses significant environmental and health hazards due to the formation of harmful byproducts:
- Carbon Monoxide (CO): A colorless, odorless, and highly toxic gas that can cause oxygen deprivation and even death.
- Soot (C): Fine particulate matter that contributes to air pollution, respiratory problems, and climate change.
- Unburned Hydrocarbons: Volatile organic compounds (VOCs) that contribute to smog formation and can have carcinogenic effects.
Minimizing incomplete combustion is, therefore, crucial for protecting both human health and the environment. This is achieved through careful control of air-to-fuel ratios in combustion systems and the use of catalytic converters to further oxidize harmful emissions.
Complete and incomplete combustion dictate not only the products of the reaction but also its efficiency and environmental impact. With a firm understanding of these pathways, we can now turn our attention to the world beyond the laboratory, examining where this fundamental process of ethane combustion plays out in our daily lives.
Real-World Applications and Implications
Ethane combustion, a seemingly simple chemical reaction, underpins a surprisingly wide array of industrial processes and has profound implications for our environment and safety protocols.
Power Generation and Industrial Heating
One of the most significant applications of ethane combustion is in power generation.
Ethane, often extracted alongside natural gas, serves as a valuable fuel source in power plants.
When combusted, it releases substantial thermal energy, which is then used to generate steam.
This steam drives turbines that produce electricity, powering homes, businesses, and entire cities.
Beyond electricity, ethane combustion is integral to industrial heating processes.
Many industries, such as chemical manufacturing and refining, require high temperatures for various operations.
Ethane combustion provides a reliable and cost-effective means of achieving these temperatures, enabling the production of essential materials and products.
Environmental Impact: Balancing Benefits and Concerns
While ethane combustion offers numerous benefits, it is crucial to acknowledge its environmental impact.
The primary concern stems from the emission of greenhouse gases, particularly carbon dioxide (CO2), a major contributor to climate change.
The complete combustion of ethane produces CO2 and water, while incomplete combustion can generate carbon monoxide (CO) and other harmful pollutants.
These emissions can contribute to air pollution, respiratory problems, and other adverse health effects.
Mitigating Environmental Concerns
To mitigate these environmental concerns, various technologies and strategies are being implemented.
Carbon capture and storage (CCS) technologies aim to capture CO2 emissions from power plants and industrial facilities, preventing them from entering the atmosphere.
Improving combustion efficiency through advanced burner designs and control systems can minimize the formation of pollutants.
Transitioning to alternative, cleaner fuels is also a critical step in reducing the environmental footprint of ethane combustion.
Safety Considerations: Handling a Flammable Substance
Ethane is a highly flammable substance, and its combustion poses certain safety risks.
Leaks and uncontrolled combustion can lead to fires and explosions, causing significant damage and potential injuries.
Therefore, strict safety protocols and procedures are essential when handling and using ethane.
Ensuring Safe Handling
Proper ventilation, leak detection systems, and fire suppression equipment are crucial for preventing accidents.
Regular inspections and maintenance of equipment, as well as comprehensive training for personnel, are also vital for ensuring safe operations.
Adherence to industry standards and regulations is paramount in minimizing the risks associated with ethane combustion.
FAQs: Mastering the Combustion of Ethane Equation
These frequently asked questions will further clarify the ethane combustion process and its associated equation.
What exactly does "complete combustion" mean in the context of the ethane equation?
Complete combustion, referring to the combustion of ethane equation, indicates that the reaction proceeds with sufficient oxygen (O2). This ensures ethane (C2H6) fully reacts to produce only carbon dioxide (CO2) and water (H2O), maximizing energy released and minimizing byproducts.
Why is it important to balance the combustion of ethane equation?
Balancing the combustion of ethane equation ensures mass conservation. This means the number of atoms of each element is the same on both sides of the equation. A balanced equation is essential for stoichiometric calculations, like predicting reactant amounts needed and product yields.
What happens if there isn’t enough oxygen during ethane combustion?
If there’s insufficient oxygen, the combustion of ethane equation leads to incomplete combustion. This results in the formation of carbon monoxide (CO), soot (C), and less energy released compared to complete combustion. Carbon monoxide is a toxic gas.
Are the coefficients in the balanced combustion of ethane equation just ratios, or do they represent absolute amounts?
The coefficients in the balanced combustion of ethane equation represent mole ratios. They indicate the relative quantities of each substance involved in the reaction. While not absolute amounts, they define the proportion needed for a complete and balanced reaction.
Alright, future chemists! You’ve tackled the combustion of ethane equation in this ultimate guide. Now go forth and impress everyone with your newfound knowledge. If you found it helpful, spread the word, and maybe even try balancing some other exciting equations!